Thought Leadership
Battery modelling – Why ‘physics-based’ is not always best
19 April 2023

Given the complex and derivative nature of the acadamic battery modelling landscape, it can be tempting to be led down a rabbit hole of ever-increasing depth while progressively losing touch with reality. It's time to wake up from Wonderland, with a pragmatic 'physics-informed' mindset for a more grounded approach to battery management.
Anna Tomaszewska
Senior Engineer - Battery Electrochemical Modelling
Tom Maull
Senior Engineer
Abstract
Models are essential for engineering efficient systems both quickly and cost-effectively. They focus the mind on what’s important and inform where our precious testing resources should be allocated. Batteries are complex, with behaviours covering a wide range of length-scales and timescales; yet a strong understanding of them pays dividends at all stages of the value chain, from materials R&D, to manufacturing, optimal control, reuse, and recycling.
But what does understanding truly mean when it comes to battery modelling to address real-world problems? The academic ‘Physics-based’ battery modelling landscape is highly derivative in nature. As industry looks to apply this research, it can be tempting to be led down a rabbit hole of ever-increasing depth while progressively losing touch with reality; overwhelmed by an information overload, one can lose track of what’s important, and forget the caveats and assumptions made along the way. In the words of Douglas Adams, “the most misleading assumptions are the ones you don’t even know that you are making”; these cautionary words of warning are especially pertinent to battery modellers. It’s time to take a step back, and ask ourselves What questions are we trying to answer?, and How should we best equip ourselves to answer them’?.
To coin a phrase ‘all models are wrong, but some are useful’, the goal of battery models is to empower battery developers & and operators to make more grounded decisions in managing their assets for ensuring long term, predictable value.
Physics-informed modelling is a mind-set that seeks to wake us up from Wonderland, bridging the gap between electrochemical science and the real-world. By hand-picking the most important aspects of the ‘physics-based’ modelling domain, we end up with powerful models that offer a window into battery safety, lifetime, and performance, without compromising the most important requirement – practical deployment – for batteries, everywhere.

The ‘physics-based’ revolution…
Battery models are essential for optimising designs, evaluating performance, and informing battery management. When used correctly, model-based workflows streamline testing. Once a model is validated, it can serve to replace the need for a new test each time a question is asked of the system. Model-based workflows are of particular benefit in battery management, as battery performance is highly dependent on the battery’s state, be it polarisations accrued over a drive cycle, or years of degradation; just as a person’s 100m sprint time will depend on whether they’ve just run a marathon, as well as their age and fitness. The state, defined by the duty cycle history, is dependent on user inputs, and therefore unpredictable. In the absence of a model-based approach power limits must be conservative to avoid erratic and reactive behaviours.
Battery models can vary significantly in form, from the simplest ‘integrator’ models, which describe the open circuit voltage dependency as the battery is charged and discharged, through to continuum scale and atomistic models describing, in detail, the journey of the lithium ion from cathode to anode.
Approaches to modelling the voltage response of the battery under usage can be broadly placed on a spectrum of data-driven, empirical, and physics-based. Data-driven models, by design, apply minimal functional constraints, relying on big-data to ‘learn’ how batteries behave. The complex history dependencies in battery behaviour, however, have led to a consensus against this type of model in isolation; they tend to overfit to training data, and therefore break down in general cases. Semi-empirical equivalent circuit networks, on the other hand, add structure, through application of established functional forms that approximate the high-level voltage dynamics of a battery under load. These models remain low order, and so are practical to parameterise, but are limited in the lack of insight into internal electrochemical states.
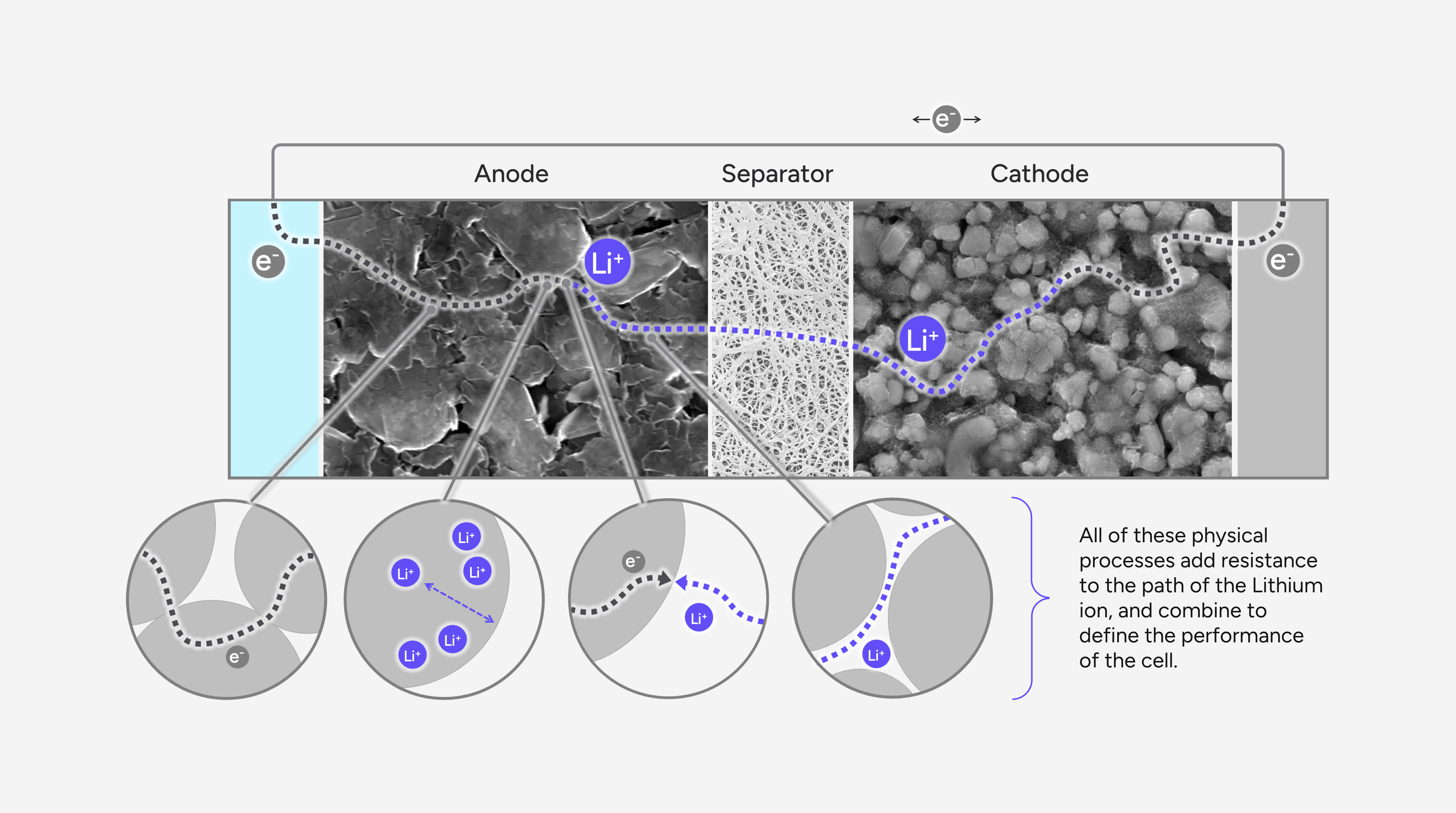
This interpretability deficit is addressed by electrochemical or ‘physics-based’ models, which aim to decompose the voltage response of the battery into its constituent physical processes in a strive for greater transparency into the key electrochemical states. These estimated states may better inform performance limitations, degradation tipping points and safety.
Physics-based battery models are derived from porous electrode theory, relying on fundamental physical principles to simulate lithium-ion transport and distribution within the electrodes and electrolyte. Constructed from first principles, they consist of coupled sets of partial differential equations, which describe the thermodynamics, reaction kinetics and the various transport processes occurring within the battery cell. Due to their white-box nature, one can explore the dynamic behaviour of active species and associated potentials throughout the electrode sandwich in a spatially resolved manner which is particularly useful for understanding the sensitivity of cell design parameters, the relative importance of manufacturing control characteristics, or simply for elucidating battery behaviours in the field.
The most misleading assumptions are the ones you don’t even know that you are making
Douglas Adams
Given all the benefits, it may seem that physics-based modelling is the only way to go. However, despite intensive academic activity in this space, industry has yet to widely incorporate these approaches into their product development and battery management workflows. Why is this?
Academic research is derivative in nature, tending towards broadening and augmenting established models, by introducing increasingly complex phenomena with the release of each new paper. These may, for example, describe novel or composite electrode materials, or address known limitations such as the assumption of uniform particle size. In practice, however, academics may tend to supplement the models while neglecting the assumptions on which they are based. Some simplifications are knowingly ignored; solid state diffusivity, a key parameter, can vary by several orders of magnitude with state of charge, current, and temperature, but is often (knowingly) approximated by a static value1’. Others are simply forgotten, such as the assumptions made when solving Butler-Volmer kinetics analytically2.
As model complexity is increased, its dimensionality increases also, which introduces a parameterisation dilemma. The gold standard, so-called pseudo-2-dimensional (P2D) model by Doyle, Fuller and Newman, has 44 physical parameters. Some of these parameters can be directly measured in the lab through cell disassembly, while others are more difficult to measure and therefore often propagated from other academic papers or inferred from experimental data. It can be shown through dimensionality reduction that the model is overparameterized, and that the number of parameters may be significantly reduced3. In doing so, however, one must lump the measurable cell parameters with those that can only be extracted by making several unrealistic assumptions, which calls into question why physical measurements are necessary in the first place. An example is the solid-state diffusion time scale, which for spherical particles can be described as the ratio between the square of particle radius and the solid-state diffusivity of the electrode material. The particle size can be measured directly through microscopy4. The typical methods of measurement of solid-state diffusivity, however, require the use of currents much lower than those encountered in most applications and assume a uniform current distribution along a planar surface, a condition inherently inapplicable to porous battery electrodes. For these reasons, diffusivity often requires adjustment by several orders of magnitude to reflect the observed voltage response. Since the two parameters are then combined into a diffusion time scale in physics-based model formulations, the manipulation of diffusivity values renders the accuracy of particle size measurement largely irrelevant.
Paradoxically, the inherent complexity drives over-simplification in key assumptions, which makes achieving an accurate voltage response while maintaining physical interpretability a difficult balance to strike. Chen’s 2020 work on the LG M50 21700 cell, which represents the state-of-the-art in physics-based model parameterisation research, demonstrates that to achieve reasonable accuracy over a range of use-cases, some of the most sensitive (and meaningful) parameters require significant adjustment from the physical measurements or literature values by up to 1800%5.
The models we apply in the real world need to be easily adapted as the battery degrades using field data. This is a problem for full-order physics-based models, as the sheer number of electrochemical states implies an impractical number of degradation modifiers, which are difficult to observe independently, even from lab data, let alone from real-world fleet data where we have just one measurement, the terminal voltage. Perhaps the most obvious example which can cause the model to break-down is that recovering both anode and cathode surface concentrations requires knowledge of both the lithium inventory and the remaining active material in each electrode, which can be difficult to observe from real-world field data.
So perhaps the reason for limited adoption is the ‘analysis paralysis’ that comes from a seemingly endless list of caveats in applying physics-based models to the real world, both theoretically, around model validity, and in practice, in their requirement for prohibitively expensive hardware when applying them in Battery Management Systems. A solution is needed to liberate the battery industry from this paradigm while harnessing the best of electrochemical science.

The pareto-principle for battery modelling
The pragmatism of ‘semi-empirical’ and the interpretability of ‘physics-based’ need not be mutually exclusive. The ideal model applies the principle of Occam’s razor through model forms that can be parameterised parsimoniously from the observations available, cutting out unnecessary assumptions. The hybrid, ‘physics-informed’ model that emerges from this exercise should be transparent enough to inform performance, lifetime, and safety, without overpromising in its physical interpretability. Simplifications should be explicit and unapologetic, justified only through a deep knowledge of the same first-principles from which the full order models are derived.
In the end, to coin a phrase ‘all models are wrong, but some are useful’, the goal of battery models is to empower battery developers and operators to make more grounded decisions in managing their assets for ensuring long term, predictable value.
The Physics-Based Model is lost, misled, misunderstood. It is wrong, but it’s also useful, provided we accept its limitations. For informing design, they are invaluable, as the validity range may be limited, and design parameter sensitivity is more important than model accuracy. However, beyond the workstation, physics-based models as we know them today are not fit for purpose. The true role for physics-based models is in validating the assumptions made in the journey towards the reduced order physics-informed models that realistically serve to replace the empirical models in use today, whilst benchmarking the interpretability compromises that are made along the way6.
1 Chen et al. (2020) describe this as a ‘huge simplification’ in ‘Development of Experimental Techniques for Parameterization of Multi-scale Lithium-ion Battery Models’.
2 Butler-Volmer reaction kinetics for describing intercalation charge transfer can be solved analytically provided the charge transfer coefficient is assumed to be 0.5, which has several implications which are easily forgotten.
3 Structural identifiability of a pseudo-2D Li-ion battery electrochemical model
4 This measurement as well is usually associated with flattening a particle size distribution to a single representative value and assuming spherical shape.
5 The maximum concentration (which describes the amount of Lithium that the electrode can accommodate) was adjusted by 20%, while the solid diffusivity (which describes the rate of diffusion of Li-ions within the electrode) was adjusted by 1800%.
6 Virtual cells can be designed for which all parameters are known, making the physics-based model a useful tool for interpretability assessment.
Read more


